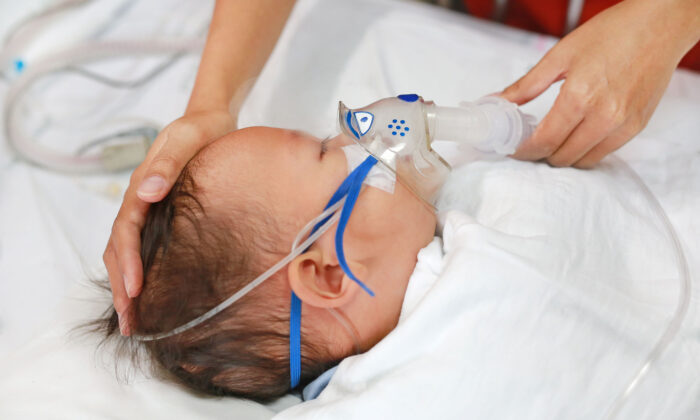
The Potential Risks and Rewards of Using Antibiotics in Infants for Immune Health
Scientists have discovered a connection between gut microbiome disruption from antibiotics and increased susceptibility to asthma.
The use of antibiotics to treat infections in babies may lead to respiratory issues later in life.
Australian Study Shows Gut-Lung Connection
The study, published in the journal Immunity, was conducted in Australia. Researchers using mice found that the molecule indole-3-propionic acid (IPA) produced by gut bacteria is significantly reduced with early-life antibiotic use.
“The microbiome and the immune system go through crucial development stages in the first year of life, and it is during this period that antibiotic treatment can predispose an individual to allergies or asthma,” explained Benjamin Marsland, a professor at Monash University’s Department of Immunology and Pathology and the lead author of the study, to The Epoch Times.
“Our research indicates that IPA plays a particularly vital role early in life, and its absence can make developing lung cells more prone to inflammation.”
- Improving blood glucose levels
- Increasing insulin sensitivity
- Correcting intestinal microbial disorders
- Inhibiting toxin penetration
- Modulating the immune system’s response
This study is the first to link IPA with protection against future asthma development, Marsland added.
Asthma is a long-term lung condition caused by airway narrowing when inflamed, and it is the most common chronic disease among children.
Inflammation of the airways plays a critical role in allergic reactions. When individuals with allergies encounter allergens, such as dust, their immune system reacts excessively. This exaggerated response triggers the release of chemicals, including histamine, leading to airway inflammation.
The study revealed that mice given antibiotics in their first year of life were more prone to airway inflammation from house dust mites. This elevated susceptibility persisted into adulthood, even after IPA levels and gut microbiome returned to normal.
However, supplementing the mice’s diet with the IPA molecule effectively prevented the development of house dust mite-induced airway inflammation, or asthma, in adulthood.
Similarly, antibiotic use has been shown to decrease IPA levels in humans, indicating that the findings of the Australian study may be relevant for human health.
“The potential use of IPA as a complementary therapy along with antibiotics in early life to counteract the negative effects of antibiotics and protect lung development is highlighted,” Marsland emphasized.
Study Warns of Caution
A separate longitudinal study, published in the International Journal of ObGyn and Health Sciences, involving 300 children aged 1 to 5 years, identified a troubling trend.
Children who received antibiotics experienced a significant reduction in gut microbiota abundance and diversity, leading to a higher incidence of respiratory and gastrointestinal infections compared to those with minimal or no antibiotic exposure.
The researchers found that antibiotic use disrupted the gut microbiome and immune regulation, increasing children’s susceptibility to pathogenic invasions. This vulnerability resulted in a cycle of recurring infections and repeated antibiotic use.
Interestingly, older children exhibited a more resilient response to antibiotic exposure.
“While there were still disruptions in their microbiota and immune functions, the effects were less severe, and the recovery period was shorter compared to younger children,” the researchers noted. “This suggests that the maturing immune system offers some level of protection against the adverse effects of antibiotics.”
The studies coincide with a report from the Australian Commission on Safety and Quality in Health Care (ACSQHC) published in August, stating that taking antibiotics for common infections has “little to no benefit.”
Antibiotics are used to treat diseases and infections caused by bacteria and should only be used when necessary to combat bacterial infections.
The ACSQHC highlighted that although antimicrobials (including antibiotics, antivirals, antifungals, and antiparasitic agents) are crucial in healthcare, the overuse of antibiotics has led to the development of antimicrobial resistance, affecting essential treatments like cancer chemotherapy, diabetes management, organ transplantation, and major surgeries.