FDA Overhaul Needed for New Vaccines and mRNA Therapies
The pandemic has ended, but the introduction of the COVID-19 vaccines which use mRNA technology, signifies the start of a new era in modern medicine. The lagging regulatory framework which the FDA cobbled together specifically for mRNA vaccine approval has set the stage for adverse events related to genetic therapies using this new technology. In this series, we will reveal emerging concerns about mRNA injections related to the lipid nanoparticles, spike protein, and vaccine contamination as public documents are released.
Summary of Series Key Facts
- According to a Moderna Securities and Exchange Commission filing in June 2020, “Currently, mRNA is considered a gene therapy product by the Food and Drug Administration (FDA).”
- The FDA created new guidance, released in June 2020, for gene therapy products to be marketed as vaccines against COVID-19. They were not put through the same testing requirements as other RNA therapeutics. The new vaccines were also not required to go through human biodistribution studies.
- Had mRNA vaccines been held to the same regulatory standards required for novel therapeutics, the following three issues would likely have been identified prior to authorization for human use:
- The lipid nanoparticle (LNP) shell used to deliver the mRNA has inflammatory potential and can cluster with other LNPs or fall apart, allowing the mRNA inside to fall out and circulate freely in the bloodstream.
- The spike protein coded by the mRNA and its S1 subunit has been found in the blood following vaccination. Both the spike protein and the S1 subunit are associated with inflammation and clotting.
- Contamination during the manufacturing process can cause impurities in the vaccine, such as mRNA fragments and bacterial plasmids. Testing by pharma before authorization found impurities—have these issues been fixed?
- Despite the promising potential of mRNA therapeutics, did the pandemic emergency provide reasonable justification for the suspension of typical regulatory requirements?
- Should these vaccines have been recommended only for the highest-risk individuals pending further human testing? Should vaccine information sheets have included all known risks to allow for full and complete informed consent?
- Were mandates unethical given the lack of standard pre-authorization safety testing?
- All of these questions are relevant given the development of new mRNA vaccines against influenza and respiratory syncytial virus (RSV). What regulatory framework will apply going forward? Will these newer mRNA vaccines be subject to stricter oversight aligned with, to borrow Moderna’s wording, “genetic therapy” or the lagging framework used for COVID-19 mRNA vaccines?
When a new vaccine is developed for humans, it is subject to rigorous safety testing—first in animals, then in humans. To illustrate what actually happened with the mRNA vaccines, let’s say the COVID-19 vaccine was the first bioengineered egg to be tested by the FDA for safe human consumption. The egg “shell” is the lipid nanoparticle (LNP) capsule that carries the genetically modified “contents,” the mRNA and spike protein.
The FDA decided to relax their regulations and only test the LNP shell in animals and bypassed testing of the contents (mRNA and spike protein) in animals or humans. This testing would have determined how the body responds to the new technology (biodistribution study).
In other words, the FDA approved the first mRNA “vaccine” injected into the human body without checking the biodistribution of the “contents” (the mRNA and spike protein) for human safety. They only checked the LNP “shell” on animals before giving their stamp of approval. Even the limited LNP testing data is alarming.
Despite this lack of adequate safety testing for the first mRNA “vaccine” used in humans, the FDA granted authorization and assured the public with authoritative certainty that the entire product was safe. As serious adverse events unfolded at an unprecedented rate, the FDA doubled down on its safety claims, requiring no additional studies.
The limited data we do have is concerning because it shows that the LNP spreads throughout the body instead of just staying in one place. This limited level of testing is not permitted for the approval of other drugs.
The European version of the U.S. FDA—the European Medicines Agency (EMA), was more open than the FDA regarding the limited data available. The EMA shared many details about how the LNP (the “shell”) spreads throughout the body. It also expressed concerns about impurities in the vaccines during manufacturing. We will discuss these issues thoroughly in this series, including excerpts from EMA reports. The Pfizer report submitted to the FDA, which we will also cover next, is only available via a Freedom of Information Request. Why is there such reluctance to share testing data?
Relaxed Regulations for COVID mRNA Vaccines
Before a new drug or vaccine is approved by a health authority, they must understand how the body will process the drug.
Typically, a non-clinical pharmacokinetics (PK) study report is submitted to the FDA to explain how the drug is released, absorbed, distributed, metabolized, and excreted from the body. This is called a biodistribution study.
However, during the COVID pandemic, the FDA modified its typical approval process for new vaccines in response to the public health emergency. The new “non-binding recommendations” for the pharmaceutical industry issued in June 2020 relaxed the rules for mRNA vaccine approval compared to what is typically required for “gene therapy.”
The new FDA guidance allowed companies to present data collected from other development platforms. In other words, studies that were conducted on other products were permitted to support the application for emergency use of the mRNA vaccines.
“COVID-19 vaccine development may be accelerated based on knowledge gained from similar products manufactured with the same well-characterized platform technology, to the extent legally and scientifically permissible. (page 3)
“In some cases, it may not be necessary to perform nonclinical safety studies prior to [first in human] FIH clinical trials because adequate information to characterize product safety may be available from other sources. For example, if the COVID-19 vaccine candidate is made using a platform technology utilized to manufacture a licensed vaccine or other previously studied investigational vaccines and is sufficiently characterized, it may be possible to use toxicology data (e.g., data from repeat dose toxicity studies, biodistribution studies) and clinical data accrued with other products using the same platform to support FIH clinical trials for that COVID-19 vaccine candidate.” (page 7)
The request for biodistribution studies is written in very general terms, without sufficient specificity for a novel therapeutic like the COVID-19 mRNA vaccines:
“Biodistribution studies in an animal species should be considered if the vaccine construct is novel in nature and there are no existing biodistribution data from the platform technology. These studies should be conducted if there is a likelihood of altered infectivity and tissue tropism or if a novel route of administration and formulation is to be used.” (page 7)
While the FDA authorized these products under these relaxed rules, other scientists have suggested more specificity was needed. For example, a review by Vervaeke and colleagues stated: “The rapid rise of mRNA therapeutics has resulted in a regulatory framework that is somewhat lagging.” They explain further in the abstract that a “multi-layered approach” should be used to understand what the new products do in vivo (in the human body). One specific guideline proposed by the authors states: “Biodistribution studies for RNA therapeutics should encompass both the RNA molecule(s), the individual components of the carrier, the combined RNA-carrier drug, and the produced protein.”
In other words, test the shell of the egg and the contents.
As we will demonstrate, adhering to such guidelines would have been very helpful prior to authorization for human use. However, to our knowledge, none of the current COVID-19 mRNA vaccines have ever been through such biodistribution studies to evaluate the RNA molecule and its encoded spike protein. Only the LNP carrier capsule has been studied in this manner, and only in animals, not humans.
FDA Review of Pfizer Biodistribution Study
Based on the FDA’s modified rules for mRNA vaccines, the agency reviewed Pfizer’s BNT162b2 LNP carrier biodistribution study report in November 2020 to understand how the mRNA vaccine would work. The report was marked as “approved” on Nov. 9, 2020, on the left side of the (pdf).
As will be presented here and in Part 2, this report was widely adopted by the European Medicines Agency, the Australian Therapeutic Goods Association, and the Japanese government.
However, the LNP is only the carrier of the vaccine mRNA (the “shell” of the “egg”); it is not the key active ingredient of the mRNA vaccine (the contents of the “egg”).
Further, the inside of the egg was replaced with a substitute—the mRNA carried in the LNP study coded for (luciferase), not the same spike-protein encoding mRNA used in the vaccine. Finally, two of the lipids used in the LNP molecule had not previously been authorized for use in humans. Thus, novel lipids were being developed to carry a novel vaccine for a mass vaccination campaign, yet no human biodistribution studies were solicited.
The FDA typically requires human studies early in drug development and as few as six healthy volunteers would have been required. Given that our bodies manufacture the spike protein once the vaccine is injected, human studies should have been done to evaluate the production, distribution, and metabolism of the mRNA and spike protein throughout the body.
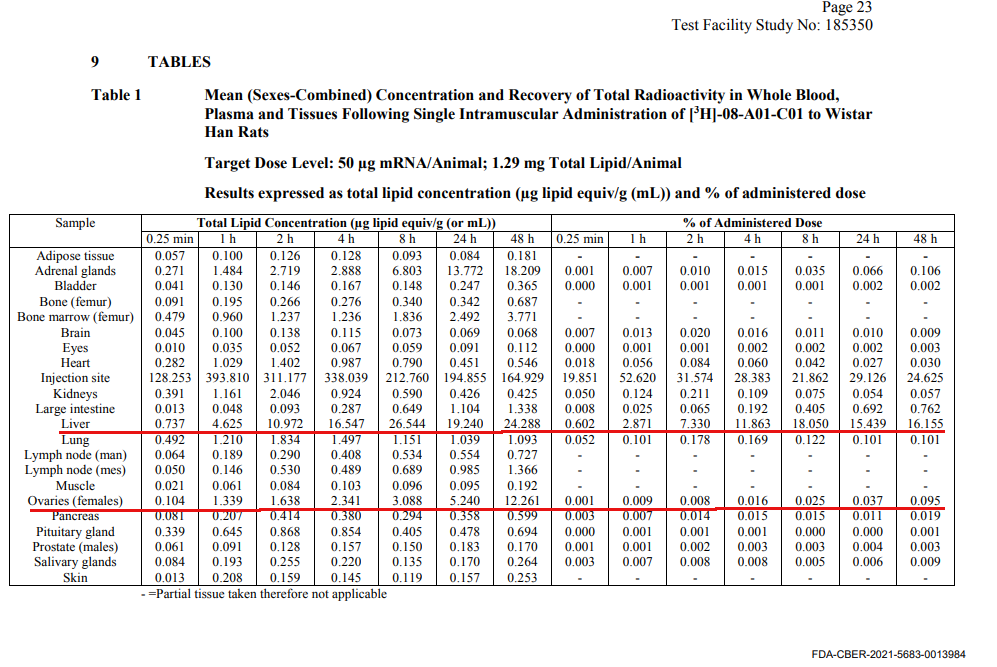
While not ideal, animal studies do reveal helpful information. The biodistribution study report submitted by Pfizer used radioactive labeling to tag the LNPs in the vaccine administered to 21 male and 21 female rats (page 9). This allowed the scientists to track and quantify the amount of vaccine reaching various organs over 48 hours following injection.
The rats were injected with 50 micrograms of mRNA vaccine. After 15 minutes, in addition to having a relatively high mRNA concentration at the injection site, the vaccine began to disperse to different organs throughout the body, reaching the liver and spleen first.
After an hour, the concentration of the vaccine in the liver and spleen increased further and it reached the adrenal glands and bone marrow.
After 24 hours, the researchers examined the distribution of the mRNA vaccine in the rats and found that in addition to the highest level being at the injection site, the next highest levels were in the spleen, liver, adrenal glands, ovaries, bone marrow, lymph nodes, kidneys, muscles, and heart, in order of the concentration. Find the number 24.288 below for the liver reading at 48 hours. This number can be found in both the Australian and Japanese reports which will be discussed in Part 2 of this series.
Figure 1: Rat Biodistribution Study Data in an FDA Report
CDC’s Silent Removal of Reassuring Message
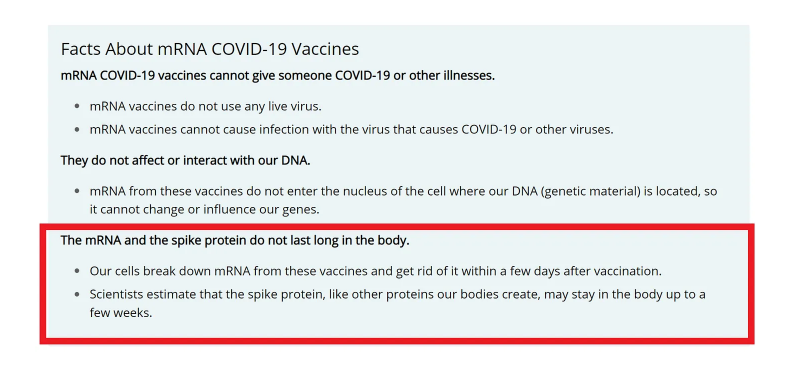
After issuing authorization, the U.S. Centers for Disease Control and Prevention (CDC) sought to calm fears about toxicity and claimed that the mRNA is broken down and removed from the body quickly, while the spike protein may take longer to clear. (Figure 2a)
Yet the claim below in the red box, which was stated on the CDC website on July 15, 2022, has been quietly removed. In so doing, does the CDC acknowledge that evidence is lacking to support these claims?
Figure 2a: CDC Reassurance That mRNA and Spike Protein Disintegrate Quickly
Sometime after July 2022, the CDC updated this web page to reassure the public that the mRNA vaccines do not integrate into the human genome, with no mention of how long the spike protein and mRNA will last in the body. (Figure 2b)
Figure 2b: CDC Reassurance That mRNA Does Not Enter the Cell Nucleus

Why did the CDC change the messaging on its website to focus on DNA integration instead of how long mRNA and spike protein last in the body?
Next: A closer look at the reports from health authorities in Australia, Japan, and Europe provide insight into why the CDC may have withdrawn this claim that the mRNA is broken down quickly and that spike protein does not linger in the body. The European Medicines Agency (EMA) reports show where the LNP shell and mRNA travel throughout the body.
◇ References
Addgene. Molecular Biology Reference.
Alana F Ogata, Chi-An Cheng, Michaël Desjardins, Yasmeen Senussi, Amy C Sherman, Megan Powell, Lewis Novack, Salena Von, Xiaofang Li, Lindsey R Baden, David R Walt, Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients, Clinical Infectious Diseases, Volume 74, Issue 4, 15 February 2022, Pages 715–718.
Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol. 2022 Feb 25;44(3):1115-1126. doi: 10.3390/cimb44030073. PMID: 35723296; PMCID: PMC8946961.
Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults N Engl J Med 2020; 383:2427-2438
Anderson S. CBER Plans for Monitoring COVID-19 Vaccine Safety and Effectiveness. October 20, 2020. Accessed 3/20/23.
Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS-CoV-2 vaccines: Lights and shadows. Eur J Intern Med. 2021 Jun;88:1-8. doi: 10.1016/j.ejim.2021.04.019. Epub 2021 Apr 30. PMID: 33966930; PMCID: PMC8084611.
Baker, A. T., Boyd, R. J., Sarkar, D., Teijeira-Crespo, A., Chan, C. K., Bates, E., Waraich, K., Vant, J., Wilson, E., Truong, C. D., Lipka-Lloyd, M., Fromme, P., Vermaas, J., Williams, D., Machiesky, L., Heurich, M., Nagalo, B. M., Coughlan, L., Umlauf, S., Chiu, P. L., … Borad, M. J. (2021). ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Science Advances. 7(49), eabl8213.
Baumeier C, Aleshcheva G, Harms D, Gross U, Hamm C, Assmus B, Westenfeld R, Kelm M, Rammos S, Wenzel P, Münzel T, Elsässer A, Gailani M, Perings C, Bourakkadi A, Flesch M, Kempf T, Bauersachs J, Escher F, Schultheiss H-P. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. International Journal of Molecular Sciences. 2022; 23(13):6940.
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther 28, 117–129 (2021).
Chauhan, H., Mohapatra, S., Munt, D.J. et al. Physical-Chemical Characterization and Formulation Considerations for Solid Lipid Nanoparticles. AAPS PharmSciTech 17, 640–651 (2016).
Chui CSL, Fan M, Wan EYF, et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: A self-controlled case series study. Lancet. 2022;(50).
Dag Berild J, Bergstad Larsen V, Myrup Thiesson E, et al. Analysis of Thromboembolic and Thrombocytopenic Events After the AZD1222, BNT162b2, and MRNA-1273 COVID-19 Vaccines in 3 Nordic Countries. JAMA Netw Open. 2022;5(6):e2217375. doi:10.1001/jamanetworkopen.2022.17375.
daSilva RL. Viral-associated thrombotic microangiopathies. Hematology/Oncology and Stem Cell Therapy. 2011:4(2):51-59.
De A, Ko YT. Why mRNA-ionizable LNPs formulations are so short-lived: causes and way-out. Expert Opin Drug Deliv. 2023 Feb;20(2):175-187. doi: 10.1080/17425247.2023.2162876. Epub 2023 Jan 1. PMID: 36588456.
Doshi P. BMJ 2021;373:n1244 Covid-19 vaccines: In the rush for regulatory approval, do we need more data? | The BMJ
Ehaideb, S.N., Abdullah, M.L., Abuyassin, B. et al. Evidence of a wide gap between COVID-19 in humans and animal models: a systematic review. Crit Care 24, 594 (2020).
European Medicines Agency Assessment Report
Faizullin D, Valiullina Y, Salnikov V, Zuev Y. Direct interaction of fibrinogen with lipid microparticles modulates clotting kinetics and clot structure. Nanomedicine. 2020 Jan;23:102098. doi: 10.1016/j.nano.2019.102098. Epub 2019 Oct 23. PMID: 31655206.
FDA. Considerations for Human Radiolabeled Mass Balance Studies—Guidance for Industry. May 2022.
FDA. Development and Licensure of Vaccines to Prevent COVID-19.
FDA-CBER-2021-5683-0013962 approved on: 09-Nov-2020. A Tissue Distribution Study of a [3H]-Labeled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following Intramuscular Administration in Wistar Han Rats. FINAL REPORT Test Facility Study No. 185350 Sponsor Reference No. ALC-NC-0552
Fertig TE, Chitoiu L, Marta DS, Ionescu VS, Cismasiu VB, Radu E, Angheluta G, Dobre M, Serbanescu A, Hinescu ME, Gherghiceanu M. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines. 2022 Jun 28;10(7):1538. doi: 10.3390/biomedicines10071538. PMID: 35884842; PMCID: PMC9313234.
Grobbelaar LM et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19 Biosci Rep (2021) 41 (8): BSR20210611.
Hou, X., Zaks, T., Langer, R. et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6, 1078–1094 (2021).
Let’s talk about lipid nanoparticles. Nat Rev Mater 6, 99 (2021).
Michieletto, D., Lusic, M., Marenduzzo, D. et al. Physical principles of retroviral integration in the human genome. Nat Commun 10, 575 (2019).
Moghimi, S. M., & Simberg, D. (2022). Pro-inflammatory concerns with lipid nanoparticles. Molecular therapy: The Journal of the American Society of Gene Therapy, 30(6), 2109–2110.
Naturally Inspired Podcast. Jessica Rose Ph.D. VAERS, Data and Truth
Ohlson J. Plasmid manufacture is the bottleneck of the genetic medicine revolution. Drug Discov Today. 2020 Oct 16;25(11):1891–3. doi: 10.1016/j.drudis.2020.09.040. Epub ahead of print. PMID: 33075470; PMCID: PMC7564888.
Perico L, Marina Morigi M, Galbusera M, et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022.
Qin, S., Tang, X., Chen, Y. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Sig Transduct Target Ther 7, 166 (2022).
Röltgen K, Nielsen SCA, Silva O. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022;185(6):1025-1040.
Schmeling, M, Manniche, V, Hansen, PR. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine. Eur J Clin Invest. 2023; 00:e13998. doi:10.1111/eci.13998
Srinivasan M, Thangaraj SR, Arzoun H. Gene Therapy – Can it Cure Type 1 Diabetes? Cureus. 2021 Dec 19;13(12):e20516. doi: 10.7759/cureus.20516. PMID: 35004071; PMCID: PMC8723777.
Trougakos IP, Terpos E, Alexopoulos H, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Cell 2022;28(7): P542-554.
Vervaeke P, Borgos SE, Sanders NN, Combes F. Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv Drug Deliv Rev. 2022 May;184:114236. doi: 10.1016/j.addr.2022.114236. Epub 2022 Mar 26. PMID: 35351470; PMCID: PMC8957368.
Wong DWL, Klinkhammer BM, Djudjaj S, Villwock S, Timm MC, Buhl EM, Wucherpfennig S, Cacchi C, Braunschweig T, Knüchel-Clarke R, Jonigk D, Werlein C, Bülow RD, Dahl E, von Stillfried S, Boor P. Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19 Patients. Cells. 2021 Jul 27;10(8):1900. doi: 10.3390/cells10081900. PMID: 34440669; PMCID: PMC8394956.
Yonker LM, Swank Z, Bartsch YC, et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023:147(11).