The Best Time to Safeguard Against Age-Related Cognitive Decline and How to Achieve It
Recent studies indicate that the aging of the brain starts in midlife—but making early lifestyle adjustments and targeted interventions can potentially avert cognitive decline.
The neurons in our brain operate similarly to a vibrant city, where each structure depends on a consistent supply of electricity for its operations. Should a brief power outage occur, systems are in place to restore functionality—leading to minimal consequences.
However, what happens if the power loss persists for months? Although emergency generators might sustain crucial services temporarily, they too would eventually falter. Water systems might freeze and burst, buildings would start to decay, and overall infrastructure would begin to fail. When power is finally restored, the damage would already be significant—the city left in disarray.
The research indicates that aging progresses in a distinct manner, with the initial phase starting in middle age and correlating with increased insulin resistance.
Just as a city can incur lasting damage if power is restored too late, the brain can reach a point where interventions lose their effectiveness. Thus, taking early action is essential.
The Aging Brain
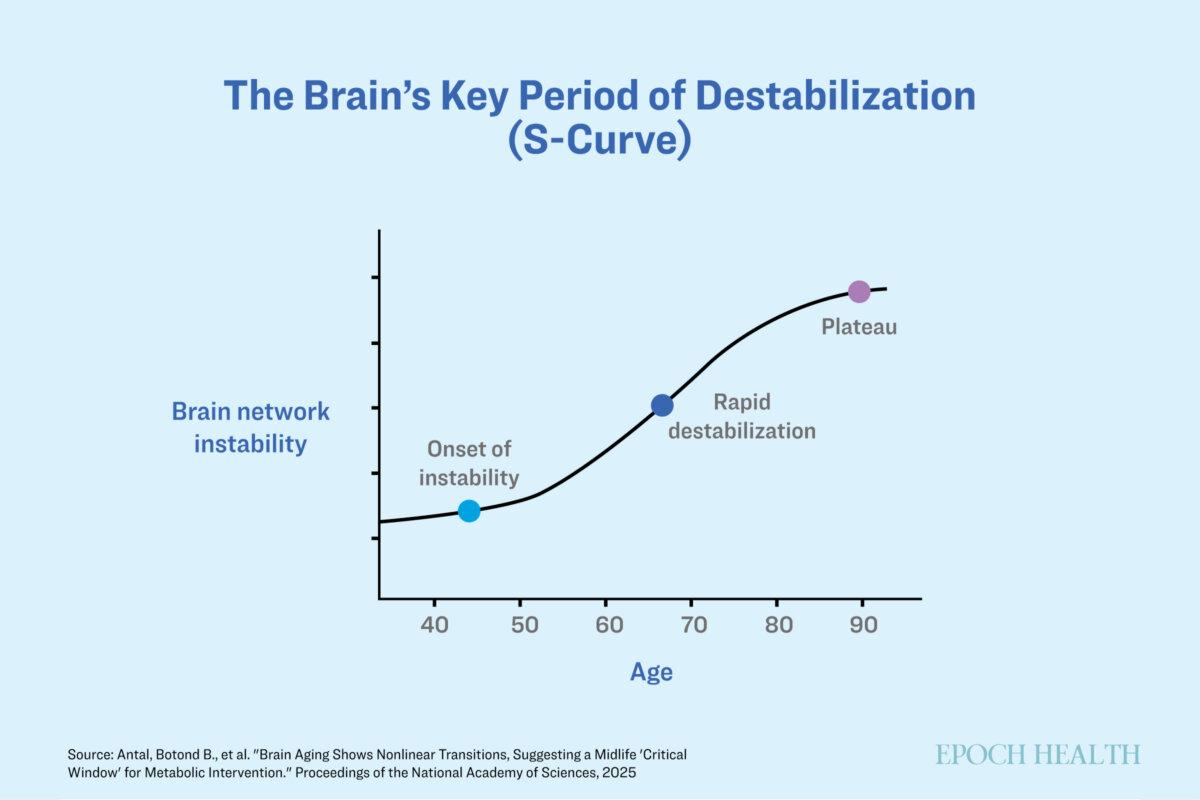
The brain experiences clear stages of degeneration—remaining stable until the mid-40s, initiating degenerative changes thereafter, and accelerating sharply by the mid-60s, according to Lilianne Mujica-Parodi, Baszucki endowed chair of Metabolic Neuroscience and director of the Laboratory for Computational Neurodiagnostics at Stony Brook University.
A critical aspect of brain aging is diminished glucose metabolism, wherein the brain struggles to efficiently utilize carbohydrates for energy, hindering its performance. These metabolic shifts commence decades prior to the manifestation of symptoms but frequently go undetected until later stages of aging, when interventions are considerably less effective. Nonetheless, techniques such as functional magnetic resonance imaging (fMRI) and electroencephalograms can identify early age-related brain alterations, presenting an opportunity for preventive measures rather than reactive treatments.
Comprehending the mechanisms of diseases is the essential first step to developing effective treatments, Mujica-Parodi noted. For instance, Alzheimer’s disease has traditionally been linked to the accumulation of beta-amyloid—a protein that forms sticky plaques between neuron cells—and tau proteins that create twisted tangles within brain cells, inspiring drug development targeting these proteins. Unfortunately, these drugs have had limited success.
A significant reason for this lack of success is that by the time Alzheimer’s is diagnosed, irreversible neuronal damage has often already taken place. The buildup of proteins is a consequence of insulin resistance in the brain; thus, focusing solely on clearing the beta-amyloid and tau proteins neglects the root cause.
In contrast to many other cell types, adult neurons possess very limited regenerative capabilities. If cognitive decline arises from neurons effectively ‘starving,’ as this study suggests, then waiting until they are incapacitated or dead is unlikely to yield effective solutions, Mujica-Parodi elaborated.
Physiological systems are engineered to sustain homeostasis—a state between energy supply and demand. A disruption in this balance can trigger stress, resulting in further dysregulation and exacerbating the issue over time, Mujica-Parodi explained.
Insulin Resistance as a Major Driver
The first major shift in the stability of brain networks coincides with increased insulin resistance, often indicated by HbA1c levels—a marker for long-term blood sugar.
Neurons rely on two main energy sources: glucose and ketones. While specific neurons require insulin to utilize glucose, those that become insulin-resistant struggle to access this energy source, leading to a condition known as “insulin resistance,” Mujica-Parodi stated.
As these cells lose the capacity to effectively use glucose—a key energy resource—metabolic stress rises, slowing communication between nerve cells and contributing to cognitive impairment.
In conditions such as Alzheimer’s disease, the uptake and processing of glucose are compromised. This is why Alzheimer’s is sometimes referred to as Type 3 diabetes, according to Angel Planells, a registered dietitian nutritionist based in Seattle.
Once neurons become insulin-resistant, they lose their capacity to utilize glucose but retain the ability to metabolize ketones—an alternative energy source that doesn’t require insulin for processing, Mujica-Parodi remarked.
Research indicates that even in older adults displaying mild cognitive impairment or Alzheimer’s, brain cells can still effectively uptake ketones; however, by this stage, substantial irreversible damage may reduce their functionality.
This underscores the necessity of identifying optimal intervention windows to proactively safeguard brain health.
Windows of Intervention
“Cognitive decline associated with aging isn’t an unavoidable part of growing older but a process that can be mitigated through early interventions aimed at reducing insulin resistance in the brain,” emphasized Mujica-Parodi.
Brain aging adheres to a predictable pattern. Rather than experiencing a gradual, linear deterioration, these changes manifest in an “S-shaped” curve, indicating specific durations when interventions may be at their most efficacious.
The average brain becomes more unstable over time, but metabolic interventions may help.The Epoch Times
The timeframe between 40 and 60 is the most crucial opportunity for intervention. During these years, brain networks experience the highest level of instability while remaining adaptable, rendering this period optimal for implementing interventions.
A Keto Diet
Metabolic interventions capable of swiftly circumventing insulin resistance have showcased effectiveness, including ketone supplementation or adherence to a ketogenic diet.
Mujica-Parodi was astonished by the rapid effectiveness of these interventions—brain networks stabilized within merely 30 minutes following the consumption of a ketone drink in her studies.
Ketones can be produced in the body through low-carbohydrate, high-fat diets or fasting—or can be supplemented; however, preserving brain health shouldn’t necessarily wait until your 40s. Early lifestyle modifications, such as embracing lower-carbohydrate, higher-fiber diets and participating in regular physical activity, can assist in preventing or postponing insulin resistance in the brain, Mujica-Parodi noted.
Upon reaching your 40s, screenings for brain insulin resistance—going beyond standard HbA1c assessments—could help detect risks early enough to enable ketogenic diets or supplements to support glucose access.
“Not everyone requires a strict keto diet,” Planells stated. “However, minimizing processed carbohydrates and enhancing insulin sensitivity can generally improve brain health.”
In addition to ketogenic diets and supplements, cognitive resilience—the brain’s capability to effectively adapt to stress and uphold its functionality—can also be cultivated through activities like mentally stimulating tasks, learning new skills, and nurturing social relationships, according to Planells. Chronic stress and elevated cortisol levels can accelerate brain aging, reinforcing the value of mindfulness practices, such as meditation.
“The window of opportunity may be narrow, but knowing it exists empowers us to take action.”





