Lawsuit Filed Against Pharmaceutical Company for Selling Ineffective Cold Medications
Sick customers who purchased certain cold and flu medications have allegedly been falsely promised relief from nasal congestion, according to an Australian law firm.
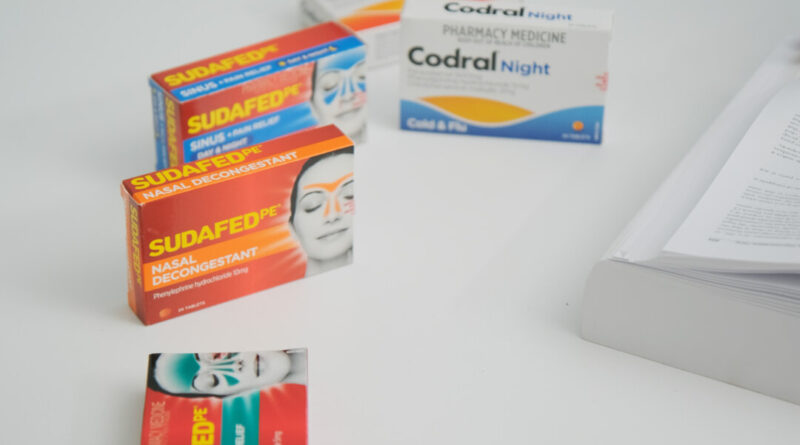
Attorneys at JGA Saddler have launched a class action lawsuit on behalf of millions of customers, claiming that they were deceived by pharmaceutical giant Johnson & Johnson’s over-the-counter medications such as Codral Day and Night, Sudafed PE, and Benadryl PE.
The lawsuit revolves around tablets containing phenylephrine, a substance that the U.S. Food and Drug Administration (FDA) has deemed ineffective as a nasal decongestant when taken in tablet form.
In 2006, Johnson & Johnson switched from pseudoephedrine to phenylephrine after the former was removed from shelves due to concerns about drug abuse.
Brisbane ear, nose, and throat specialist Jo-Lyn McKenzie stated that phenylephrine is not a decongestant.
“It’s unethical for corporations to sell healthcare products while aware that they are ineffective,” Dr. McKenzie remarked.
JGA Saddler director Rebecca Jancauskas insisted that Johnson & Johnson must be held responsible.
“Johnson & Johnson has produced and promoted a medication that multiple years of evidence have demonstrated does not function as advertised, utilizing outdated, unreliable studies to market products to the Australian public that do not deliver on their promises,” she explained.
“Australians trusted these products to perform as promised and would not have purchased them if they knew they were ineffective at alleviating congestion.”
The law firm encourages other impacted customers to reach out and participate in the class action.
Johnson & Johnson has been reached out to for a comment.